This example will demonstrate how to calculate the compressibility of real gas in order to determine gas density and specific gravity at a specific condition.
Calculate the following based on the given condition:
1) Density of this gas under the reservoir conditions of 7,500psia and 220ºF,
2) Specific gravity of the gas.
Gas component is shown in Table 1
Clik here to view.

Table 1 – Gas Component
Average density of air = 28.96 lb/cu-ft
Solution
- Determine critical pressure and temperature of gas mixtures using Kay’s rule
Clik here to view.

Table 2 – Critical Pressure and Temperature
Note: critical pressure and temperature can be found from this link – http://www.drillingformulas.com/determine-compressibility-of-gases/
Pc’ = Σyipci = 660.5 psia
Tc’ = ΣyiTci = -46.2 F = -46.2 +460 F = 413.8 R
Clik here to view.

Table 3 – Pc’ and Tr’ by Kay’s Rule
- Calculate Tr and Pr
Tr = T ÷Tc
Tr = (220+460) ÷ (-46.2+460)
Tr = 1.64
Note: temperature must be in Rankin.
Rankin = Fahrenheit + 460
For the critical temperature calculation, it can be converted the critical temperature from F to R before calculating Tc’. This will still give the same result.
Pr = P ÷ Pc
Pr = 7500 ÷ 660.5 = 11.4
- Read the compressibility factor (z) from the chart.
z = 1.22
Clik here to view.

Figure 1-z-factor from the Standing and Katz Chart
- Calculate average molar mass
Average Molar Mass = Σyi×Mi = 22.1 lb
Clik here to view.

Table 4 – Average Molar Mass of Gas
- Calculate density of gas from the equation below;
Image may be NSFW.
Clik here to view.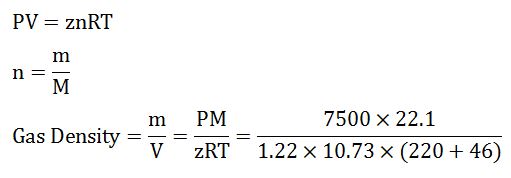
Gas Density = 18.6 lb/cu-ft
- Calculate gas specific gravity from the equation below;
SG = Gas Density ÷ Air Density
SG = 18.6 ÷ 28.96
SG = 0.64
Summary:
The answers for this answer are listed below;
Gas Density = 18.6 lb/cu-ft
SG = 0.64
We wish that this example will help you understand to determine z-factor and use it to calculate any related information.
References